Electricity is responsible for the radio system operation. It allows the radio signal to be emitted at the transmitter, which is in turn captured by the receptor, and is interpreted to control the rudder and carb servos.
The device responsible for providing this electricity is the battery � a set of cells wired together that stores and supplies the demanded electricity.
The batteries used in R/C belong to one of those groups:
conventional: batteries that can�t be recharged when empty and because of this they are discarded after use; alkaline batteries belong to this group;
rechargeable: as the name implies, those are batteries that can be recharged after use and, because of this, have a longer useful life. Nickel Cadmium (NiCad) and NickelMetalHidride (NiMH) are examples.
Although there might be some controversy about the issue, rechargeable batteries are more and more often used in the R/C field and for that reason the text below deals, exclusively, with this type. Moreover it deals specifically with the rechargeable batteries used in the radio system, not taking in account Lithium Ion or Lithium Polymer, which practical use has yet to be proven. Conventional or rechargeable batteries of another type are only mentioned when, for the sake of clarity, comparisons are made in the text. Additionally, the segment tries to bring information to the r/c boater who needs to know what to do, how take care of his batteries and how to make good use of them. We try to avoid excessive technical language and scientific explanations over what happens in the battery inwards, except when this was essential to the understanding. Should you feel the urge for an in deep analysis � including the scientific reason of the why and how things happen � look for the links on my links page. There are technical papers from experts available at the web.
The two most important characteristics of a battery are the tension it supplies and it�s capacity.
Tension: it is the voltage that the battery is able to supply under demand, measured, obviously in volts: 4,8V, 6V, 8,4V, etc. The bigger the voltage supplied to an electric motor, the faster it turns, until the point where it�s limit is exceeded and the motor burns. That is the reason why our servo responds better and faster when feed by 6 batteries over a 4,8V.
Note: all modern servos can be feed by 6V batteries without problems.
Capacity: indicated in mili ampere/hour (mAH) it is the time that the battery is able to supply the tension necessary to operate the system to which is wired. A 1.000 mA battery is able to supply a 1.000 mA current for 1 hour, or 500 mA for 2 hours or 2.000 for 0.5 hours.
So, the battery voltage should be the one that the devices it will be wired to are designed for � over this limit fatal damage may occur. As for capacity, the bigger the better, more running time for the system with no collateral effects. The limit is the battery size because for bigger capacity at the same battery we have a bigger size. Soon we are dealing with a battery so big that the additional running time is not justified. In r/c boats AA cells are standard and even with this limitations each year more capacity batteries are marketed.
By the way, what is a cell?
It is each one of the components that wired to each other make the battery. Each AA cell is capable of supplying 1,2V � so we need 4 cells to get a 4,8V battery (4 x 1,2) and 5 to get 6V (5 x 1,2).
To reach this voltage the cells are wired in series � positive pole to the negative of the next, the positive of that to the negative of the other, and so on. When cells are wired in series, the final voltage is the sum of the individual voltages and the capacity is the capacity of one single cell.
In other way, in a parallel wiring, all the positives poles are wired together, and the same is true for all the negative poles. Is this type of connection, the final voltage is the voltage of one single cell and the capacity is the sum of the capacities of all cells. Results that a battery of 5 cells with 1.2V and 1.000 mA will have 1,2V and 5.000 mA if wired in parallel and 6V and 1.000 mA if wired in series.
As our radio system operates on 4,8V or 6V, parallel wiring is not used in our systems. Besides, NiCad cells have a very low internal resistance and wiring in parallel cells with different capacities results in electricity flowing from the cell with bigger capacity to the one with less, heating the wires and even risking an explosion.
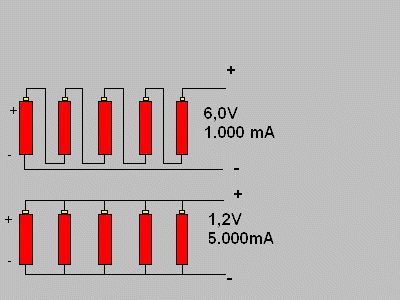
The drawing bellow shows the wiring of 5 cells, each one with 1,2V and 1.000 mA and makes things clear. The battery that comes from this has 6V and 1.000 mA in the series wiring and 1,2V and 5.000 mA in parallel.
The next items compare specific characteristics between NiCad and NiMH, the most common used on R/C.
Nickel Cadmium:
favorable cost/benefit;
longer cycle-life (charge/discharge);
great performance under load;
easily recharged;
forgiving to abuse;
can be quick charged;
low self discharge rate (discharge that happens to a battery not in use)
Nickel Metal Hydride:
30% more capacity when compared to a standard NiCad
memory effect less pronounced;
less demanding to exercise (discharge/charge) to maintain peak performance;
internal components environment friendly.
The table below resumes those characteristics, comparing both; in bold face the preferred one.
| Type | NiCad | NiMH |
| energy density (Wh/kg) | 50 | 75 |
| cycle life | 1.500 | 500 |
| fast charge time | 1.5 h | 2/3 h |
| self discharge | medium | high |
| load current | very high | medium |
| cycle life | 30 dias | 90 days |
| memory effect | bigger | smaller |
| cost | smaller | bigger |
As you may see, NiCad batteries have some better characteristics over NiCad ones: greater capacity and less memory effect. Nevertheless, and far from what is commonly believed, memory effect does exist, although in a lesser rate. The battery needs to be recycled less frequently to maintain peak performance. However, in all other characteristics the NiCad are superior: life, recharging time, self discharge, current under load and, last but not least, cost. Once more, you are your own judge. What we can tell about the issue is that we, at Shark Racing, already used both types and, honestly, couldn�t find any advantage on the NiMH that justifies the higher price.
Memory effect:
The cycle life of a rechargeable battery shortens with time, because of the memory effect, which makes an even full charged battery not being able to operate at full capacity when demanded. The name comes from the time where nickel cadmium batteries �remembered� how much they have been discharged the last time in use and had a tendency to only accept charge to that limit. So, not using the battery to full capacity lessened, at each new recharge, the current capacity it was able to store. This is not true anymore to moderns NiCad batteries but the name survived. What happens to those batteries is the growing of the nickel and cadmium crystals they are made of whenever they are slow charged for a long time or frequently recharged without being completely discharged, what should be done periodically. In the limit, the crystal growing may even result in puncture of the separator, rendering the battery useless.
Normally, the memory effect on NiCad batteries may be eliminated by recycling the battery (discharge the battery to 1 V per cell and full recharging after). Repeated once a month, the memory effect disappears. On NiMH batteries, 3 months is a reasonable interval.
Nevertheless, it may occur that a simple recycling would be not enough to recover the battery. In this instance, recondition could be in order: a slow discharge to 0,6V per cell, under control to avoid the reverse polarity � situation in which the battery is discharged beyond its capacity and one or more cells never more recover their capacity, rendering the whole pack useless.
Self discharge:
Phenomenon inherent to all NiCad or NiMH batteries, where the battery discharges even without use. A NiCad battery looses 10% of its nominal capacity a day, which is acceptable. A problem occurs when, within this time lag, the battery full discharges. This can be caused by bad quality separators normally found on cheap batteries, bad quality charges that �boil� the battery or just plain old age. Any battery that looses more than 30% of its capacity a day is irrecoverable and should be discarded.
Care with your batteries:
The control over your boat depends on how good your battery is. Be sure they have full charge before wiring them to the radio. The transmitter has a visible indication of remaining charge that shows, at each moment, how the battery is. On the boat the situation is more complex � checking the battery in rest is not enough. The correct way of doing this is using a voltmeter in parallel with the battery poles and checking for voltage drop when the servos operate. A full charged battery should have 6V; any drop to 5V or less means a battery that demands recharging.
Remember that a NiCad battery has a discharge flow quite different from an alkaline or even other rechargeable ones: after recharge it can show a voltage a little over the nominal (1,4V is not uncommon) and when in use this voltage drops quickly to the nominal value (1,2V). From there on, the voltage drops slowly until the battery limit is attained, when the voltage drops very fast to values that prevent the battery to continue to be used.
This characteristic, if allows the battery to be used till the end of it�s capacity with the same performance, on the other hand gives no notice of a battery that needs immediate recharge.
The solution is keeping your batteries with full charge at the beginning of the day at the pond and bringing extra batteries if you suppose the day could be long.
The working voltage at the NiMH battery starts dropping at the very moment it is being used, what allows the R/C boater �seeing� that his battery is discharged, at the cost of a performance that is lessening from the first moment.
More than ever, it is a matter of personal choice between having integral performance during all use time � at the cost of more precaution on use � or not having to worry about this and dealing with a battery which performance is dropping since the very first moment of use.
Personally, and as a question of cost and habit, we, at Shark Racing, use NiCad batteries and a Fail Safe and we have had not a single problem since then.
To determine the capacity of your batteries with the resources you may have, cycle it, full charge it and after it cools off wire to it a 6V automotive bulb with 6W. This bulb will take 1A/h from the battery (6W/6V). Have a voltmeter in parallel with the bulb and check how long the battery takes to reach 1V per cell. The real capacity of your battery will be given by the equation:
A=t/C
where:
A=battery capacity in mA
t=time the battery took to drop to 1V per cell, measured in decimal:
1 hour=1
� hour=0.5
1H:45�=1,75, etc.
C=load supplied by the battery (in our example, 1A)
Trickle
Keeping your batteries when not in use plugged to a trickle charge charger assures that they will be with full charge whenever you need them. Nothing magic, just a permanent charge of 1/100 of the nominal battery capacity, that prevents self-discharge and memory effect.
4,8V or 6V:
Servos are electric motors. As already stated, electric motors run faster and with more torque when feed with higher voltage. All modern servos withstand quite well the bigger voltage and have better performance under those circumstances. As important as the maximum voltage the motor can withstand is the minimum voltage they run with. Obviously, if a servo can operate between 4,8V and 6V, the voltage bellow that it is not capable of doing the job (operate the rudder and carb servos) is under the lower limit. A 6V battery may operate the servos even when its voltage reduced 20%, to 5V. At the same condition, a 4,8V battery would have only 3,84V, not enough to operate the servos.
Final remarks:
After all that have been told, some conclusions and recommendations may be stated:
1. considering the cost/benefit and other factors involved, the NiCad battery is still the better choice;
2. cycle your batteries periodically;
3. always use the correct charger, recommended by the battery manufacturer;
4. run away from cheap batteries � the quality is awful, cells are mismatched and certainly future problem are not worth the economy;
5. even if your batteries may be recharged with fast charge, it is recommended that you slow charge them after 6 fast charges.
Glossary:
energy density: watts (volts x amperes) available for each kilogram of battery weight (Wh/kg).
cycle life: number of times the battery may be discharge/charged before reducing its capacity to 80% of the original capacity � the bigger number means a battery with more useful life;
fast time charge: time a discharged battery takes to be full recharged and be ready for use on a high current charge � a small number means a battery that more quickly recovers it�s original condition to use;
self discharge: time the battery takes to full discharge when not in use � the bigger number means a battery that takes longer to discharge (medium means a battery that looses 1 to 2% of it�s capacity a day);
load current: maximum current the battery may provide under demand � the bigger number means a battery with better capacity to attend the need of the devices wired to it. The unit which measures this discharge level is C, equivalent to the total nominal battery capacity � so, a 1.000mA battery discharged at 1C will be providing a 1.000mA current, at 0,5 C a current of 500mA and at 2 C a 2.000mA current. A battery with a very high load current has a C bigger than 1, a high one a C equals to 1 and a medium load current a C smaller than 1.
exercise requirement: frequency the battery needs to be recycled (discharge/charged) to maintain peek performance;
matched cells: a battery where all the cells have the same capacity; this kind of battery is more efficient because mismatched cells discharge at different rates and the ones with low capacity discharge first, reducing the total voltage the battery is able to provide. Ideally, the battery should have matched cells not only regarding discharge time but also to charging time, otherwise some cells will be charged first will be overcharged till the end of the process or, with some types of charger, the charging process is interrupted and the battery is not fully charged.
current-limiting chargers: a charger that keeps the current constant during all the charge process, floating the voltage;
trickle charge: a very small charge applied to the battery after the normal charge that takes care of the battery self discharge;
voltage-limiting charger: a charger that limits the voltage to a pre selected level but reduces the current while maintaining this limit.

